The American Ceramic Society recently penned an article about research that could create affordable solar fuel cells, technology that produces fuel from solar power instead of electricity.
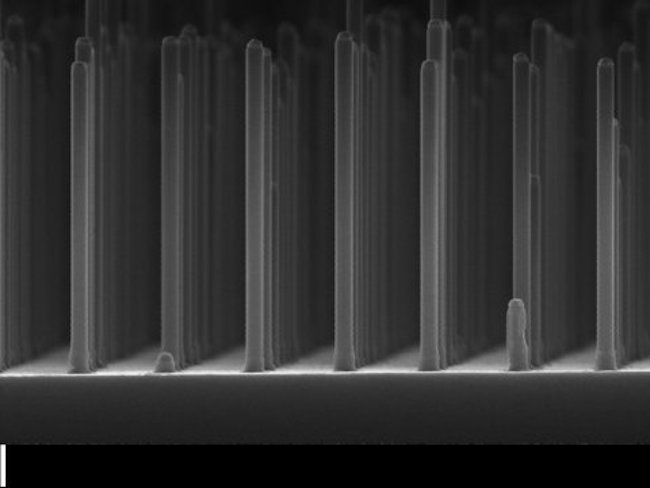
Above image: An electron micrograph of an array of gallium phosphide nanowires. Photograph from Nature Communications.
The research was published this summer by the Eindhoven University of Technology. Scientists there created a prototype cell that uses gallium phosphide nanowires to produce hydrogen gas fuel from liquid water. They report that this innovation has a yield for such fuels that is ten times greater than other cells. The design also uses less material than other cells.
Researchers state of the project:
The electricity produced by a solar cell can be used to set off chemical reactions. If this generates a fuel, then one speaks of solar fuels – a hugely promising replacement for polluting fuels. One of the possibilities is to split liquid water using the electricity that is generated (electrolysis). Among oxygen, this produces hydrogen gas that can be used as a clean fuel in the chemical industry or combusted in fuel cells – in cars for example – to drive engines.
Solar fuel cell
To connect an existing silicon solar cell to a battery that splits the water may well be an efficient solution now but it is a very expensive one. Many researchers are therefore targeting their search at a semiconductor material that is able to both convert sunlight into an electrical charge and split the water, all in one; a kind of ‘solar fuel cell’. Researchers at TU/e and FOM see their dream candidate in gallium phosphide (GaP), a compound of gallium and phosphide that also serves as the basis for specific colored leds.
A tenfold boost
GaP has good electrical properties but the drawback that it cannot easily absorb light when it is a large flat surface as used in GaP solar cells. The researchers have overcome this problem by making a grid of very small GaP nanowires, measuring five hundred nanometers (a millionth of a millimeter) long and ninety nanometers thick. This immediately boosted the yield of hydrogen by a factor of ten to 2.9 percent. A record for GaP cells, even though this is still some way off the fifteen percent achieved by silicon cells coupled to a battery.
The Ceramic society puts this work in context:
How important is developing a liquid form of solar fuel? Our current energy infrastructure is already built for it.
The Solar Fuels Institute (SOFI) at Northwestern University (Evanston, Ill.)—a global consortium leading the way in solar energy innovation—says “transportation, storage, and dispensing practices are well established for liquid fuels. Moving from a fossilized carbon-based fuel to a synthetic carbon-neutral fuel would require little to no modification of our current fuel infrastructure,” SOFI explains in a recent blog post.
Love contemporary ceramic art + design? Let us know in the comments.
Add your valued opinion to this post.